Details
BlueEasy Prestained Protein Ladder
The BlueEasy Prestained Protein Ladder is a three color protein standard with 10 prestained proteins. It has the largest range of molecular weights from 6.5 to 270 kDa. The BlueEasy Prestained Protein Ladder is designed for monitoring protein separation during SDS-polyacrylamide gel electrophoresis, verification of Western transfer efficiency on membranes (PVDF, nylon or nitrocellulose) and for approximating the size of proteins.
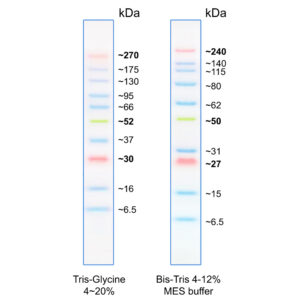
Figure 1: Molecular weight of the marker bands when using different buffer systems.
The molecular weight of the marker bands can vary depending on the gel/buffer system used. This is due to factors of the gel/buffer system that affect the running behaviour of proteins, e.g. migration rate, gel composition, pH-value (see FAQ for more information).
- Broad range: 6.5 – 270 kDa
- High performance: 10 bands with 3 colours (blue, orange and green)
- Ready to use: Supplied in a loading buffer for direct loading on gels.
- Easy to identify: The 52 (green), 30 kDa and 270 kDa (orange) are reference bands.
- Sharp bands
Contents
Approximately 0.1~0.4 mg/ml of each protein in the buffer (62,5 mM Tris-phosphate, pH 7.5 at 25°C), 2 % SDS, 10 mM Dithiothreitol, 1 mM EDTA, and 33 % (v/v) Glycerol).
500 µL Protein Marker in loading buffer are sufficient for 100 mini gels or 50 large gels.
Storage
Stable for up to 2 weeks at 25°C. Stable for up to 3 months at 4°C. For long term storage, store at -20°C.
Loading
3 μl or 5 μL per loading for clear visualization during electrophoresis on 15-well or 10-well mini-gel, respectively.
Applications
• Monitoring of protein migration during SDS-polyacrylamide gel electrophoresis.
• Monitoring of protein transfer onto membranes during Western blotting.
• Sizing of proteins on SDS-polyacrylamide gels and Western blots.
FAQ
Does this marker contain Formaldehyde or Formamide?
No.
Why are the molecular weights of the marker bands for the protein markers given separately for different systems?
There are several factors of gel and buffer chemistry that have an influence on the running behaviour of proteins. The most important are:
Migration rate: The migration of proteins in electrophoresis is influenced by the properties of the buffer system, e.g. by the ionic strength, the pH value and the presence of certain ions. The different buffer systems influence the electrophoretic mobility of proteins differently. For example, proteins may migrate faster or slower in one buffer system than in another, resulting in obvious differences in their molecular weight.
Gel composition: The type of polyacrylamide gel and its pore size can influence protein migration. Different buffer systems may be optimised for different gel types, which affects how proteins move through the gel matrix. These differences can lead to variations in the observed molecular weights.
pH: The pH of the electrophoresis buffer can also affect the charge and conformation of the proteins, which in turn affects their migration in the gel. Different buffer systems may have different pH values, leading to variations in molecular weight estimates.
Tris-glycine gels contain a moving front of Cl- ions (from the Tris-HCl in the gel) and negatively charged glycine ions from the running buffer. The glycine ions move slower than the chloride ions through the low pH (pH 6.8) collection gel and form a narrow zone where the proteins are concentrated. When the moving front meets the separating gel with the higher pH (pH 8.8), the glycine ions move quickly past the chloride ions, pulling the proteins through the gel with them, so to speak. Because of this composition, tris-glycine gels work in the strongly alkaline range and reach a pH of up to 9.5 when running.
Only logged in customers may leave a review.









Reviews
There are no reviews yet.